Symbolforklaring
| ISO 15223-1 | |
 |
Producent |
 |
Autoriseret repræsentant i Det Europæiske Fællesskab |
 |
Autoriseret repræsentant |
 |
Autoriseret repræsentant |
 |
Autoriseret repræsentant |
 |
Autoriseret repræsentant |
 |
Autoriseret repræsentant |
 |
Fremstillingsdato |
 |
Udløbsdato |
 |
Partinummer |
 |
Katalognummer |
 |
Importør |
 |
Serienummer |
 |
Modelnummer |
 |
Steril |
 |
Steriliseret med en aseptisk behandlingsteknik |
 |
Steriliseret vha. ethylenoxid |
 |
Steriliseret med stråling |
 |
Steriliseret med damp eller tørvarme |
 |
Må ikke resteriliseres |
 |
Ikke-steril |
 |
Må ikke anvendes, hvis pakken er beskadiget. Se brugsanvisningen |
 |
Steril væskebane |
 |
Steril væskebane |
 |
Steril væskebane |
 |
System med enkelt steril barriere |
 |
System med dobbelt steril barriere |
 |
Enkelt sterilt barrieresystem med beskyttende emballering indvendigt |
 |
Enkelt sterilt barrieresystem med beskyttende emballering udvendigt |
 |
Forsigtig, kan gå i stykker |
 |
Holdes væk fra sollys |
 |
Holdes tør |
 |
Laveste temperaturgrænse |
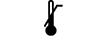 |
Højeste temperaturgrænse |
 |
Temperaturbegrænsning |
 |
Grænse for luftfugtighed |
 |
Grænse for atmosfærisk tryk |
 |
Biologiske risici |
 |
Må ikke genbruges |
 |
Se brugsanvisningen |
 |
Se brugsanvisningen |
 |
Forsigtig |
 |
Indeholder (spor af) naturgummilatex |
 |
Indeholder humant blod eller plasmaderivater |
 |
Indeholder et medicinsk stof |
 |
Indeholder biologisk materiale af animalsk oprindelse |
 |
Indeholder biologisk materiale af human oprindelse |
 |
Indeholder farlige stoffer |
 |
Til brug flere gange på én enkelt patient |
 |
Væskebane |
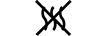 |
Ikke pyrogen |
 |
Envejsventil |
 |
Patientidentifikation |
 |
Institution eller læge |
 |
Dato |
 |
Medicinsk udstyr i henhold til EU-lovgivning |
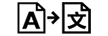 |
Oversættelse |
 |
Unik udstyrsidentifikation |
| ISO 7000/IEC 60417 | |
 |
Kan genanvendes |
| IEC 60601-1 | |
 |
Vekselstrøm |
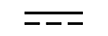 |
Jævnstrøm |
 |
Jordledning |
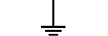 |
Jordforbindelse (jordstik) |
 |
Ækvipotentiale |
 |
KLASSE II-udstyr |
 |
“TIL” (strøm) |
 |
"FRA" (strøm) |
 |
"TIL"/"FRA" (tryk-tryk) |
 |
"TIL" for en del af udstyret |
 |
"FRA" for en del af udstyret |
 |
Type BF anvendt del |
 |
Type CF anvendt del |
 |
Kategori AP-udstyr |
 |
Farlig spænding |
 |
Defibrilleringssikker type BF anvendt del |
 |
Defibrilleringssikker type CF anvendt del |
 |
Standby |
 |
Generelt advarselsskilt |
 |
Advarsel, elektricitet |
 |
Skub ikke |
 |
Sid ikke her |
 |
Overfladen må ikke betrædes |
 |
Følg brugsanvisningen |
 |
Følg brugsanvisningen |
| IEC 60601-1 | |
 |
Beskyttet mod faste fremmedlegemer på 50 mm i diameter og derover |
 |
Beskyttet mod faste fremmedlegemer på 12,5 mm i diameter og derover |
 |
Beskyttet mod faste fremmedlegemer på 12,5 mm i diameter og derover Beskyttelse mod lodret faldende vanddråber |
 |
Beskyttet mod faste fremmedlegemer på 2,5 mm i diameter og derover |
 |
Ikke beskyttet |
 |
Beskyttelse mod lodret faldende vanddråber |
 |
Beskyttelse mod lodret faldende vanddråber, når indkapslingen hælder op til 15° |
 |
Beskyttet mod vandstænk |
| ISO 27185 | |
 |
Pacemaker, enkelt kammer, højre ventrikulær |
 |
Pacemaker, enkelt kammer, højre atrial |
 |
Pacemaker, dobbelt kammer, højre atrial, højre ventrikulær |
 |
Implanterbar kardioverter-defibrillator, enkelt kammer, højre ventrikulær |
 |
Implanterbar kardioverter-defibrillator, dobbelt kammer, højre atrial, højre ventrikulær |
 |
Pacemaker til kardial resynkroniseringsterapi, højre atrial, højre ventrikulær, venstre ventrikulær |
 |
Defibrillator til kardial resynkroniseringsterapi, højre atrial, højre ventrikulær, venstre ventrikulær |
 |
Implanterbar anordning |
 |
Implanterbar anordning (belagt) |
 |
Tilslutningstype |
 |
Momentnøgle, implanterbar implusgenerator |
 |
Åbnes her |
| ASTM F 2503 | |
 |
MR-sikker |
 |
MR-betinget |
 |
MR-usikker |
| IEC TR 60878 | |
 |
Indvendig diameter |
 |
Udvendig diameter |
| Symbol | Title | Reference | Description |
|---|---|---|---|
| ISO 15223-1: 5.1. Manufacture | |||
 |
Manufacturer | 5.1.1. | Indicates the medical device manufacturer |
 |
Authorized representative in the European Community | 5.1.2. | Indicates the authorized representative in the European Community / European Union. |
 |
Date of manufacture | 5.1.3. | Indicates the date when the medical device was manufactured. |
 |
Use-by date | 5.1.4. | Indicates the date after which the medical device is not to be used. |
 |
Lot number | 5.1.5. | Indicates the manufacturer’s batch code so that the batch or lot can be identified. |
 |
Catalog number | 5.1.6. | Indicates the manufacturer’s catalogue number so that the medical device can be identified. |
 |
Serial number | 5.1.7. | Indicates the manufacturer’s serial number so that a specific medical device can be identified. |
| ISO 15223-1: 5.2. Sterility | |||
 |
Sterile | 5.2.1. | Indicates a medical device that has been subjected to a sterilization process. |
 |
Sterilized using ethylene oxide | 5.2.3. | Indicates a medical device that has been sterilized using ethylene oxide. |
 |
Sterilized using irradiation | 5.2.4. | Indicates a medical device that has been sterilized using irradiation. |
 |
Sterilized using steam or dry heat | 5.2.5. | Indicates a medical device that has been sterilized using steam or dry heat. |
 |
Do Not Resterilize | 5.2.6. | Indicates a medical device that is not to be resterilized. |
 |
Non-Sterile | 5.2.7. | Indicates a medical device that has not been subjected to a sterilization process. |
 |
Do not use if package is damaged and consult instructions for use | 5.2.8. | Indicates a medical device that should not be used if the package has been damaged or opened. |
 |
Sterile fluid path | 5.2.9. | Indicates the presence of a sterile fluid path within the medical device in cases when other parts of the medical device, including the exterior, might not be supplied sterile. |
 |
Sterile fluid path | 5.2.9. A.12, NOTE 1 |
A.12 Examples of use of symbol 5.2.9 for “Sterile fluid path” NOTE 1: Medical device contains a sterile fluid path that has been sterilized using ethylene oxide |
 |
Sterile fluid path | 5.2.9. A.12, NOTE 2 |
A.12 Examples of use of symbol 5.2.9 for “Sterile fluid path” NOTE 2: Medical device contains a sterile fluid path that has been sterilized using irradiation. |
 |
Single sterile barrier system | 5.2.11. | Indicates a single sterile barrier system. |
 |
Double sterile barrier system | 5.2.12. | Indicates two sterile barrier systems. |
 |
Single sterile barrier system with protective packaging inside | 5.2.13. | Indicates a single sterile barrier system with protective packaging inside |
 |
Single sterile barrier system with protective packaging outside | 5.2.14. | Indicates a single sterile barrier system with protective packaging outside |
| ISO 15223-1: 5.3. Storage | |||
 |
Fragile, handle with care | 5.3.1. | Indicates a medical device that can be broken or damaged if not handled carefully. |
 |
Keep away from sunlight | 5.3.2. | Indicates a medical device that needs protection from light sources. |
 |
Keep dry | 5.3.4. | Indicates a medical device that needs to be protected from moisture. |
 |
Lower limit of temperature | 5.3.5. | Indicates the lower limit of temperature to which the medical device can be safely exposed. |
 |
Upper limit of temperature | 5.3.6. | Indicates the upper limit of temperature to which the medical device can be safely exposed. |
 |
Temperature limit | 5.3.7. | Indicates the temperature limits to which the medical device can be safely exposed. |
 |
Humidity limitation | 5.3.8. | Indicates the range of humidity to which the medical device can be safely exposed. |
 |
Atmospheric pressure limitation | 5.3.9. | Indicates the range of atmospheric pressure to which the medical device can be safely exposed. |
| ISO 15223-1: 5.4. Safe use | |||
 |
Biological risks | 5.4.1. | Indicates that there are potential biological risks associated with the medical device. |
 |
Do not re-use | 5.4.2. | Indicates a medical device that is intended for one single use only. |
 |
Consult instructions for use | 5.4.3. | Indicates the need for the user to consult the instructions for use. |
 |
Consult instructions for use | 5.4.3. A.16 NOTE | A.16 Example of use of symbol 5.4.3, “Consult instructions for use” for an electronic instruction for use (eIFU) NOTE: The eIFU indicator can be a manufacturer’s website URL or some other appropriate indication that the instructions for use are available in an electronic format. |
 |
Caution | 5.4.4. | To indicate that caution is necessary when operating the device or control close to where the symbol is placed, or to indicate that the current situation needs operator awareness or operator action in order to avoid undesirable consequences. |
 |
Contains or presence of natural rubber latex | 5.4.5. | Indicates the presence of dry natural rubber or natural rubber latex as a material of construction within the medical device or the packaging of a medical device. |
 |
Contains human blood or plasma derivatives | 5.4.6. | Indicates a medical device that contains or incorporates human blood or plasma derivatives. |
 |
Contains a medicinal substance | 5.4.7. | Indicates a medical device that contains or incorporates a medicinal substance |
 |
Contains biological material of animal origin | 5.4.8. | Indicates a medical device that contains biological tissue, cells, or their derivatives, of animal origin |
 |
Contains biological material of human origin | 5.4.9. | Indicates a medical device that contains biological tissue, cells, or their derivatives, of human origin |
 |
Contains hazardous substances | 5.4.10. | Indicates a medical device that contains substances that can be carcinogenic, mutagenic, reprotoxic (CMR), or substances with endocrine disrupting properties |
 |
Single patient multiple use | 5.4.12. | Indicates a medical device that may be used multiple times (multiple procedures) on a single patient |
| ISO 15223-1: 5.6. Transfusion/infusion | |||
 |
Fluid path | 5.6.2. | Indicates the presence of a fluid path. |
 |
Non-pyrogenic | 5.6.3. | Indicates a medical device that is non-pyrogenic. |
 |
One-way valve | 5.6.6. | Indicates a medical device with a valve that allows flow in only one direction. |
| ISO 15223-1: 5.7. Others | |||
 |
Patient identification | 5.7.3. | Indicates the identification data of the patient |
 |
Health care centre or doctor | 5.7.5. | To indicate the address of the health care centre or doctor where medical information about the patient may be found |
 |
Date | 5.7.6. | To identify the date that information was entered or a medical procedure took place |
 |
Medical device | 5.7.7. | Indicates the item is a medical device (under EU Legislation) |
 |
Translation | 5.7.8. | To identify that the original medical device information has undergone a translation which supplements or replaces the original information |
 |
Unique Device Identifier | 5.7.10. | Indicates a carrier that contains Unique Device Identifier information |
| ISO 7000/IEC 60417 | |||
 |
Recyclable | 7000 - 1135 | To indicate that the marked item or its material is part of a recovery or recycling process. |
| IEC 60601-1: General Symbols | |||
 |
Alternating current | Table D1, Symbol 1 | To indicate on the rating plate that the equipment is suitable for alternating currentonly; to identify relevant terminals. |
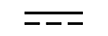 |
Direct current | Table D.1, Symbol 4 | To indicate on the rating plate that the equipment is suitable for direct currentonly; to identify relevant terminals. |
 |
Protective earth (ground) | Table D.1, Symbol 6 | To identify any terminal which is intended for connection to an external conductorfor protection against electric shock in case of a fault, or the terminal of a protectiveearth (ground) electrode. |
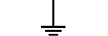 |
Earth (ground) | Table D.1, Symbol 7 | To identify an earth (ground) terminal in cases where neither the symbol 5018 nor 5019 is explicitly required. |
 |
Equipotentiality | Table D.1, Symbol 8 | To identify the terminals which, when connected together, bring the various parts of an equipment or of a system to the same potential, not necessarily being the earth (ground) potential, e.g. for local bonding. |
 |
CLASS II equipment | Table D.1, Symbol 9 | To identify equipment meeting the safety requirements specified for Class II equipment according to IEC 61140. |
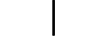 |
“ON” (power) | Table D.1, Symbol 12 | To indicate connection to the mains, at least for mains switches or their positions, and all those cases where safety is involved. |
 |
“OFF” (power) | Table D.1, Symbol 13 | To indicate disconnection from the mains, at least for mains switches or their positions, and all those cases where safety is involved. |
 |
“ON” for part of equipment | Table D.1, Symbol 16 | To indicate the ""ON"" condition for a part of equipment, if the symbol 5007 cannot be used, for example, to identify the ""ON"" position of a switch. |
 |
“OFF” for part of equipment | Table D.1, Symbol 17 | To indicate the ""OFF"" condition for a part of equipment, if the symbol 5008 cannot be used, for example, to identify the ""OFF"" position of a switch. |
 |
Type BF applied part | Table D.1, Symbol 20 | To identify a type BF applied part complying with IEC 60601-1. |
 |
Type CF applied part | Table D.1, Symbol 21 | To identify a type CF applied part complying with IEC 60601-1. |
 |
CATEGORY AP equipment | Table D.1, Symbol 22 | To identify category AP equipment complying with IEC 60601-1 which also specifies the way in which this symbol has to be used. |
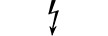 |
Dangerous voltage | Table D.1, Symbol 24 | To indicate hazards arising from dangerous voltages. |
 |
Defibrillation-proof type BF applied part | Table D.1, Symbol 26 | To identify a defibrillation-proof type BF applied part complying with IEC 60601-1. |
 |
Defibrillation-proof type CF applied part | Table D.1, Symbol 27 | To identify a defibrillation-proof type CF applied part complying with IEC 60601-1. |
 |
Stand-by | Table D.1, Symbol 29 | To identify the switch or switch position by means of which part of the equipment is switched on in order to bring it into the stand-by condition, and to identify the control to shift to or to indicate the state of low power consumption. |
 |
General warning sign | Table D.2, Symbol 2 | To signify a general warning |
 |
Warning, electricity | Table D.2, Symbol 3 | To warn of electricity |
 |
No pushing | Table D.2, Symbol 5 | To prohibit pushing against an object |
 |
No sitting | Table D.2, Symbol 6 | To prohibit sitting on a surface |
 |
No stepping on surface | Table D.2, Symbol 7 | To prohibit stepping onto a surface |
 |
Follow instructions for use | Table D.2, Symbol 10 | Symbol must appear in color. Limited to Medical Electrical Equipment covered by EN 60601-1 |
 |
Follow instructions for use | ISO 15223-1: 5.4.3. A.16 NOTE | NOTE: The eIFU indicator can be a manufacturer’s website URL or some other appropriate indication that the instructions for use are available in an electronic format. |
| IEC 60601-1; | |||
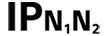 |
Table D.3, Symbol 2 | Degrees of protection provided by enclosures N1 = 0 Non-protected 1 Protected against solid foreign objects of 50 mm and greater 2 Protected against solid foreign objects of 12,5 mm and greater 3 Protected against solid foreign objects of 2,5 mm and greater 4 Protected against solid foreign objects of 1,0 mm and greater 5 Dust-protected 6 Dust-tight N2 = 0 Non-protected 1 Protection against vertically falling water drops 2 Protection against vertically falling water drops when ENCLOSURE tilted up to 15° 3 Protected against spraying water 4 Protected against splashing water 5 Protected against water jets 6 Protected against powerful water jets 7 Protected against the effects of temporary immersion in water 8 Protected against the effects of continuous immersion in water NOTE When a characteristic numeral is not required to be specified, it is replaced by the letter “X” (“XX” if both numerals are omitted). |
|
 |
Protected against solid foreign objects of 12,5 mm and greater | ||
 |
Protected against solid foreign objects of 12,5 mm and greater Protection against vertically falling water drops | ||
 |
Protected against solid foreign objects of 2,5 mm and greater | ||
 |
Non-protected | ||
 |
Protection against vertically falling water drops | ||
 |
Protection against vertically falling water drops when ENCLOSURE tilted up to 15° | ||
 |
Protected against splashing water | ||
| ASTM F 2503; Magnetic Resonance | |||
 |
MR Safe | ASTM F2503-20: 7.3.1 | An item that poses no known hazards resulting from exposure to any MR environment. MR Safe items are composed of materials that are electrically nonconductive, nonmetallic, and nonmagnetic.(ASTM F2503-20:3.1.13) |
 |
MR Conditional | ASTM F2503-20: 7.3.2 | An item with demonstrated safety in the MR environment within defined conditions including conditions for the static magnetic field, the time-varying gradient magnetic fields and the radiofrequency fields. (ASTM F2503-20:3.1.11) |
 |
MR Unsafe | ASTM F2503-20: 7.3.3 | An item which poses unacceptable risks to the patient, medical staff or other persons within the MR environment. (ASTM F2503-20:3.1.14) |