ROTAPRO™
Rotational Atherectomy System
Easy to Use. Hard on Calcium. ROTAPRO Rotational Atherectomy System is the gold standard mechanism of action on an easy to use platform.
Explore
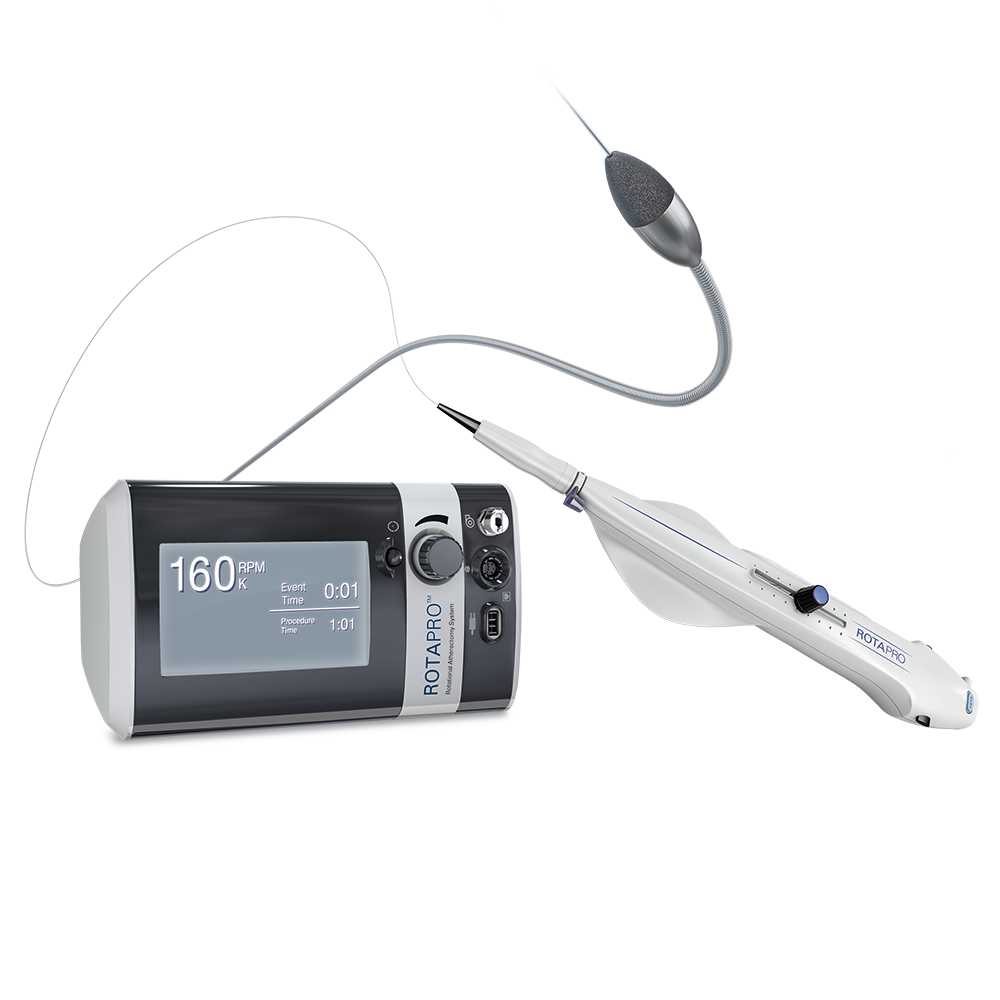
System Components
Console
- Vibrant Digital Display – Enhanced feedback and deceleration indicator
- Streamlined Connections – Quick and easy setup
- IV Pole Clamp – Installation flexibility
Advancer
- Easy-to-use Controls – Incorporated on the advancer
- Ergonomic Dynaglide™ Design – Simple system removal
- Hybrid Harness – Improved cable management
Burrs
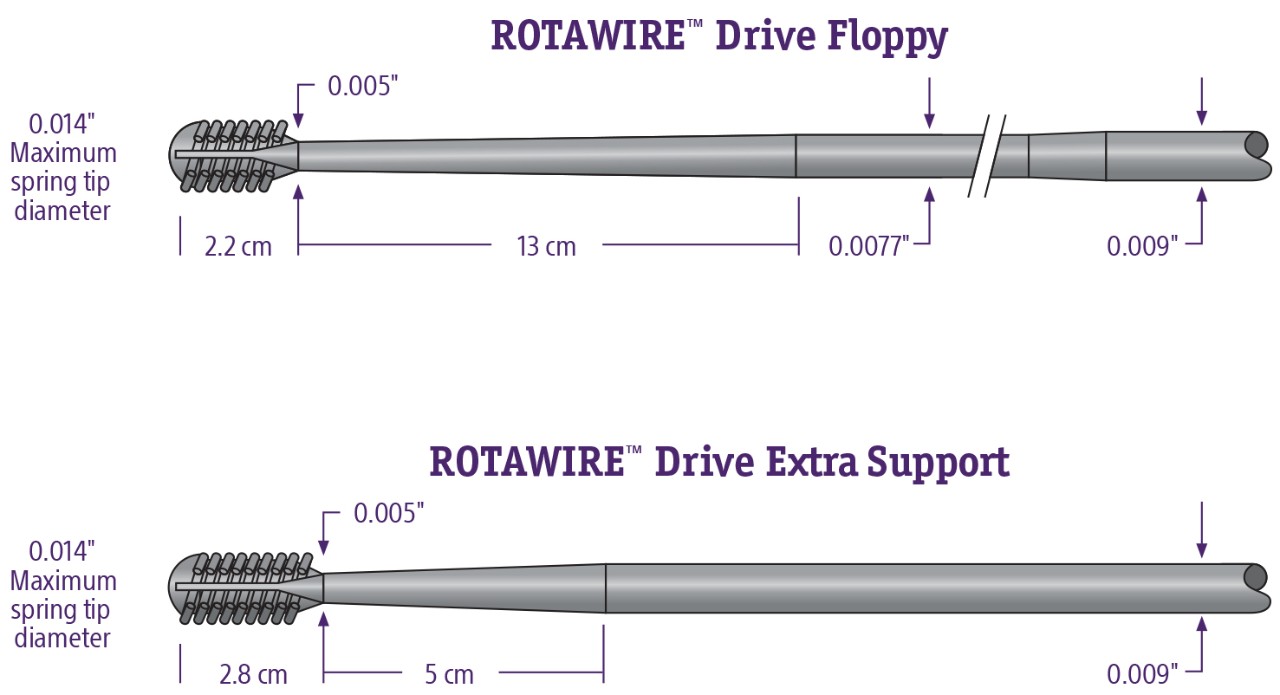
Guidewires
- Steerability – able to navigate calcified lesions with 1:1 torque through tortuous anatomy, providing access for ROTAPRO Rotational Atherectomy System
- Tip Design - .014” platinum coil to provide visibility during Rotation atherectomy
- Core Wire – one-piece core wire with Asahi technology, transmits torque for predictable steering
Choose between the ROTAWIRE™ Drive Floppy or ROTAWIRE™ Drive Extra Support models depending on procedure requirements.
ROTAGLIDE is a lipid based emulsion designed to lubricate the ROTAPRO System.*
- Reduces friction and improves tactile feel
- Reduces heat build-up around the ROTAPRO burr
- Reduces sudden drops in RPMs caused by lesion feedback
*Contraindicated if patient is allergic to eggs or olive oil
Ordering Information
ROTAPRO System Console
| Order Number | Model/ Description |
|---|---|
| H749 3930901 0 | ROTAPRO Console Kit |
ROTAPRO Pre-Connected Burr and Advancing Device
| Order Number | Model/Description | Burr Size | Length | Maximum Diameter |
|---|---|---|---|---|
| H749 39467125 0 | ROTAPRO Pre-Connected Burr and Advancing Device | 1.25 mm | 135 cm | 0.58 inch |
| H749 39467150 0 | ROTAPRO Pre-Connected Burr and Advancing Device | 1.5 mm | 135 cm | 0.58 inch |
| H749 39467175 0 | ROTAPRO Pre-Connected Burr and Advancing Device | 1.75 mm | 135 cm | 0.58 inch |
| H749 39467200 0 | ROTAPRO Pre-Connected Burr and Advancing Device | 2.0 mm | 135 cm | 0.58 inch |
| H749 39467215 0 | ROTAPRO Pre-Connected Burr and Advancing Device | 2.15 mm | 135 cm | 0.58 inch |
| H749 39467225 0 | ROTAPRO Pre-Connected Burr and Advancing Device | 2.25 mm | 135 cm | 0.58 inch |
| H749 39467238 0 | ROTAPRO Pre-Connected Burr and Advancing Device | 2.38 mm | 135 cm | 0.58 inch |
| H749 39467250 0 | ROTAPRO Pre-Connected Burr and Advancing Device | 2.5 mm | 135 cm | 0.58 inch |
ROTAWIRE™ Drive Guidewire
| Order Number | Model/ Description | Length | Tip Length | Flexibility | Spring Tip Diameter | Maximum Diameter | Quantity |
|---|---|---|---|---|---|---|---|
| H749 3946300 5 | ROTAWIRE™ Drive Extra Support Guide Wire with WireClip™ Torquer | 330 cm | 2.8 cm | Stiff | 0.009 inch | 0.014 inch | Box of 5 |
| H749 3946200 5 | ROTAWIRE™ Drive Floppy Guide Wire with WireClip™ Torquer | 330 cm | 2.2 cm | Flexible | 0.009 inch | 0.014 inch | Box of 5 |
Accessories
| Order Number | Model/Description | Quantity |
|---|---|---|
| H802 21600003 1 | Replacement Braided Air Hose | Single |
| H749 3936801 0 | Replacement Power Cord – North America | Single |
| H802 1590101 1 | Regulator Kit – North America | Single |
| H802 22196003 2 | WireClip Torquer | Box of 5 |
ROTAGLIDE™ Lubricant
| Order Number | Model/Description | Quantity |
|---|---|---|
| H749 23548-001 62 | ROTAGLIDE Lubricant Mixture 20 cc vials | Box of 6 |
Reimbursement
The C-code used for this product is C1724, catheter, transluminal atherectomy, rotational. The ROTAWIRE™ Drive Guidewire C-code is C1769. C-codes are used for hospital outpatient device reporting for Medicare and some private payers.
Note: Boston Scientific is not responsible for the correct use of codes on submitted claims; this information does not constitute reimbursement or legal advice.