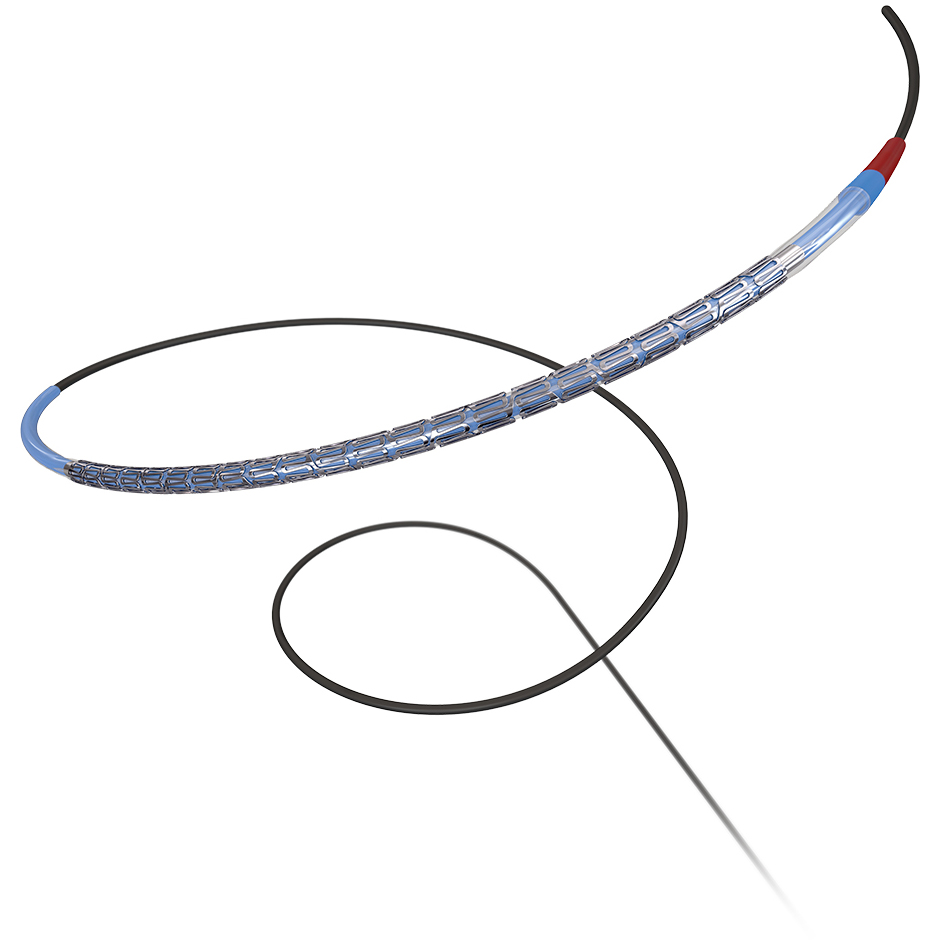
Promus ELITE™
Everolimus-Eluting Platinum Chromium Coronary Stent System
Outstanding Acute Performance. Proven Long-Term Outcomes.
The Promus ELITE Stent System builds on the proven performance of the Promus permanent polymer DES family and the platinum chromium (PtCr) platform with a new enhanced delivery system for outstanding deliverability and acute performance.
Key Resources
Explore
Product Details
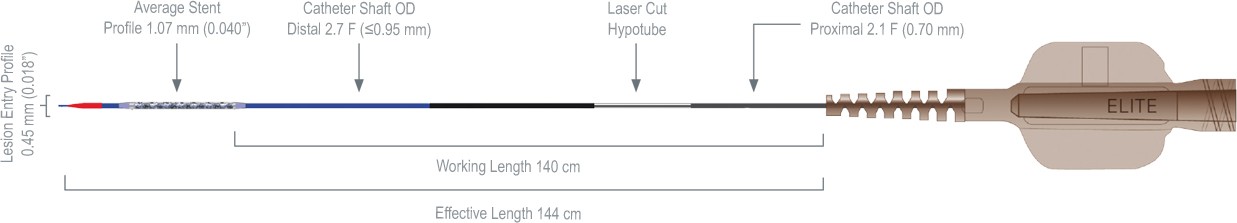
Ordering Information
| (mm) | 8 | 12 | 16 | 20 | 24 | 28 | 32 | 38 |
| 2.25 | H7493941208220 | H7493941212220 | H7493941216220 | H7493941220220 | H7493941224220 | H7493941228220 | H7493941232220 | n/a |
| 2.50 | H7493941208250 | H7493941212250 | H7493941216250 | H7493941220250 | H7493941224250 | H7493941228250 | H7493941232250 | H7493941238250 |
| 2.75 | H7493941208270 | H7493941212270 | H7493941216270 | H7493941220270 | H7493941224270 | H7493941228270 | H7493941232270 | H7493941238270 |
| 3.00 | H7493941208300 | H7493941212300 | H7493941216300 | H7493941220300 | H7493941224300 | H7493941228300 | H7493941232300 | H7493941238300 |
| 3.50 | H7493941208350 | H7493941212350 | H7493941216350 | H7493941220350 | H7493941224350 | H7493941228350 | H7493941232350 | H7493941238350 |
| 4.00 | H7493941208400 | H7493941212400 | H7493941216400 | H7493941220400 | H7493941224400 | H7493941228400 | H7493941232400 | H7493941238400 |
Clinical Information
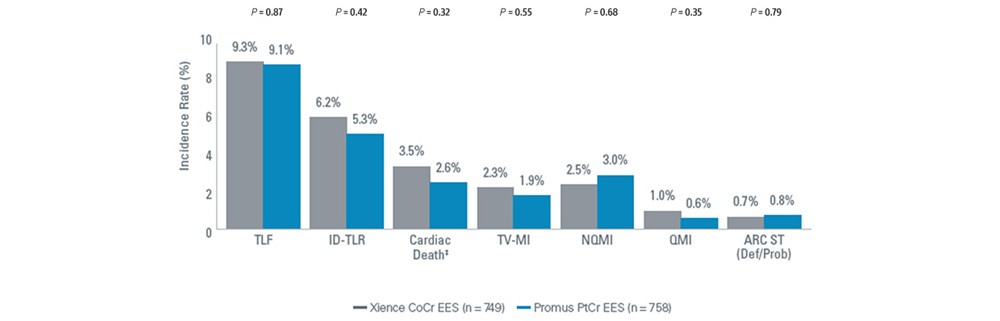
PLATINUM Workhorse Trial
Study Objective: Evaluate the safety and effectiveness of the Promus PtCr EES Coronary Stent System2 for the treatment of patients with up to 2 de novo lesions ≤ 24 mm in length; ≥ 2.50 mm to ≤ 4.25 mm in diameter compared to the Xience CoCr EES.
Study Design: Prospective, Randomized, Controlled, Non-inferiority, Multicenter
Numerically Lower Event Rates1 | 5-Year Results
Reimbursement
The C-Code used for the Promus ELITE Everolimus-Eluting Platinum Chromium Coronary Stent System is C1874 Stent, coated/covered with delivery system. C-Codes are used for hospital outpatient device reporting for Medicare and some private payers.
Note: Boston Scientific Corporation is not responsible for correct use of codes on submitted claims; this information does not constitute reimbursement or legal advice.
Tools and Resources
-
 May 30, 2018
May 30, 2018Latex Information: Promus ELITE Everolimus-Eluting Platinum Chromium Coronary Stent System
Downloadable latex information letter for the Promus ELITE Stent System. PDF, 170.0 KB
-
 May 30, 2018
May 30, 2018Metal Composition: Promus ELITE Everolimus-Eluting Platinum Chromium Coronary Stent System
Downloadable metal composition letter for the Promus ELITE Stent System. PDF, 175.0 KB
-
 May 30, 2018
May 30, 2018MRI Safety: Promus ELITE Everolimus-Eluting Platinum Chromium Coronary Stent System
Downloadable MRI safety letter for the Promus ELITE Stent System. PDF, 183.0 KB