EMINENT clinical trial results
EMINENT1 is the largest Randomized Controlled Trial (2:1) comparing Eluvia™ Drug-Eluting Vascular Stent System to self-expanding bare metal stents (BMS) for SFA/PPA; EU multi-center; superiority trial; core lab adjudicated.
EMINENT RCT 1-year primary patency results
Eluvia demonstrated superiority over BMS2 with a statistically significant primary patency of 85.4% versus 76.3% through 1-Year.
1-Year Kaplan-Meier primary patency estimate
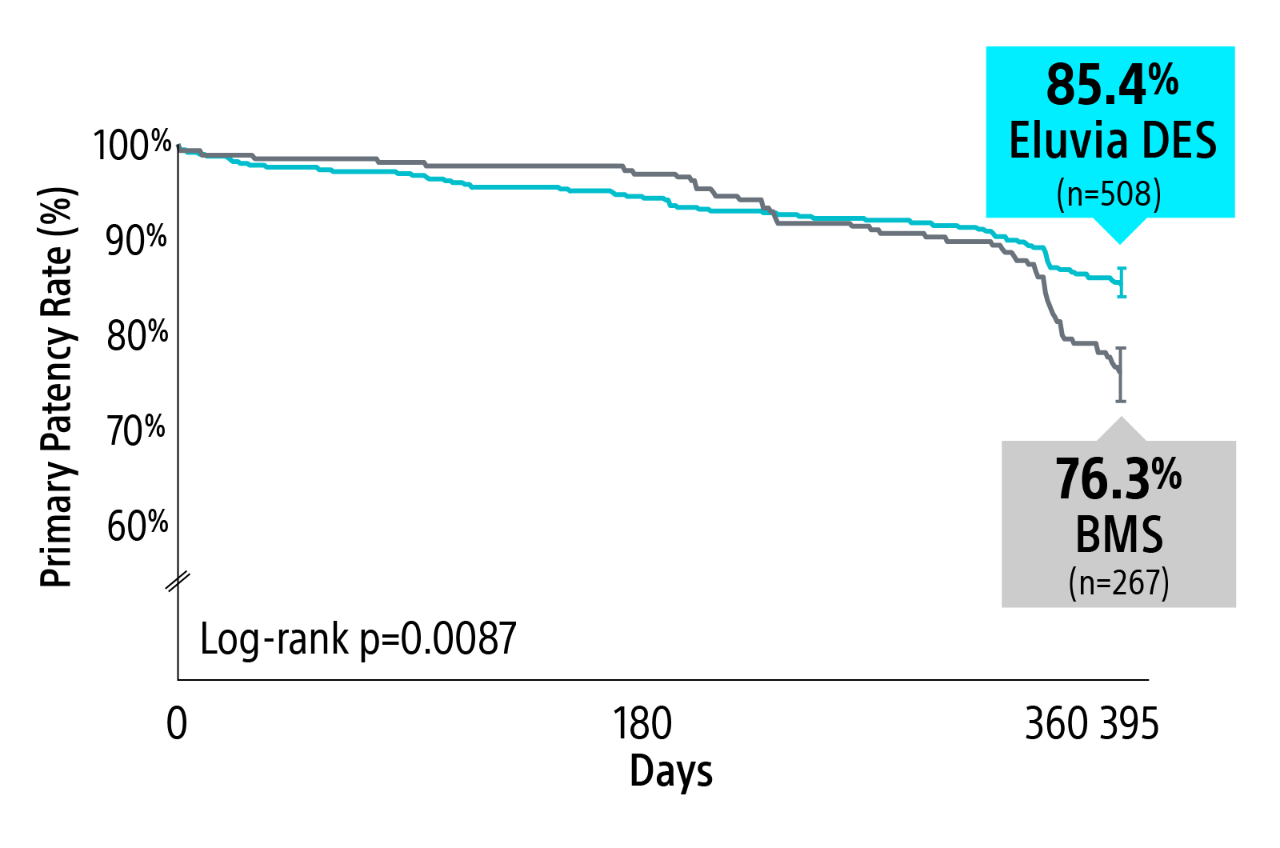
Kaplan-Meier Estimate: Primary patency defined as core-lab assessed duplex ultrasound peak systolic velocity ratio (PSVR) ≤ 2.4 at 1-year in the absence of clinically-driven TLR or bypass of the target lesion.
Log-rank p-value compares the entire K-M curves from time point zero to day 395 (full 1-year follow-up window).
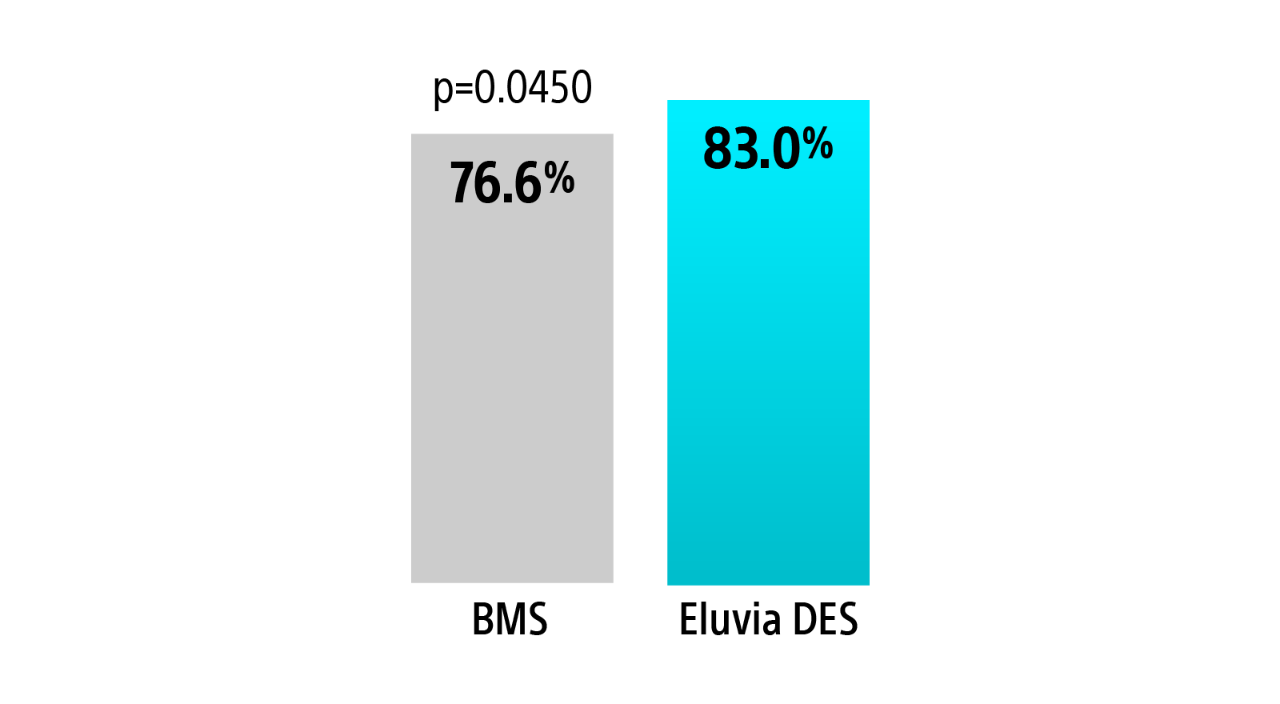
EMINENT RCT 1-year primary sustained clinical improvement
Eluvia demonstrated a statistically significant greater rate of sustained clinical improvement without reintervention over BMS through 1-Year.3
EMINENT randomized controlled trial details
- 1-year safety results
- Baseline characteristics
| Eluvia DES (n=492) |
BMS (n=273) |
p-value | |
| All Death, Major Amputation, TLR | 11.8% (56/474) | 11.8% (31/263) | 0.9912 |
| All-Cause Death at 1-Year | 2.7% (13/474) | 1.1% (3/263) | 0.1528 |
| Target Limb Major Amputation | 0.2% (1/474) | 0.0% (0/263) | 1.0000 |
| Clinically-Driven Target Lesion Revascularization | 8.4% (40/474) | 10.6% (28/263) | 0.3212 |
| Eluvia DES (n=508) |
Control (n=267) |
p-value | |
| Age (Years) | 68.9 ± 8.7 | 68.9 ± 9.1 | 0.9739 |
| Male Gender | 71.5% | 67.4% | 0.2431 |
| Diabetes Mellitus (medically-treated) | 31.9% | 32.6% | 0.8440 |
| History of Smoking (current/previous) | 36.0% / 39.6% | 36.0% / 41.6% | 0.9849 / 0.5884 |
| Percent Stenosis (%) | 86.6 ± 15.2 | 85.5 ± 15.3 | 0.3629 |
| Total Occlusions | 42.3% | 39.9% | 0.5372 |
| Total Stented Length (mm) | 105.8 ± 48.4 | 109.2 ± 49.8 | 0.3858 |
| Target Lesion Length (mm) | 75.6 ± 50.3 | 72.2 ± 47.0 | 0.3815 |
| Moderately Calcified | 21.6% | 26.0% | 0.1849 |
| Severely Calcified | 30.3% | 31.1% | 0.8122 |