COMPARE randomized controlled trial results
COMPARE is the World’s First Head-to-Head Prospective, RCT (1:1) directly comparing SFA DCBs – low dose Ranger DCB vs higher dose IN.PACT DCB.1
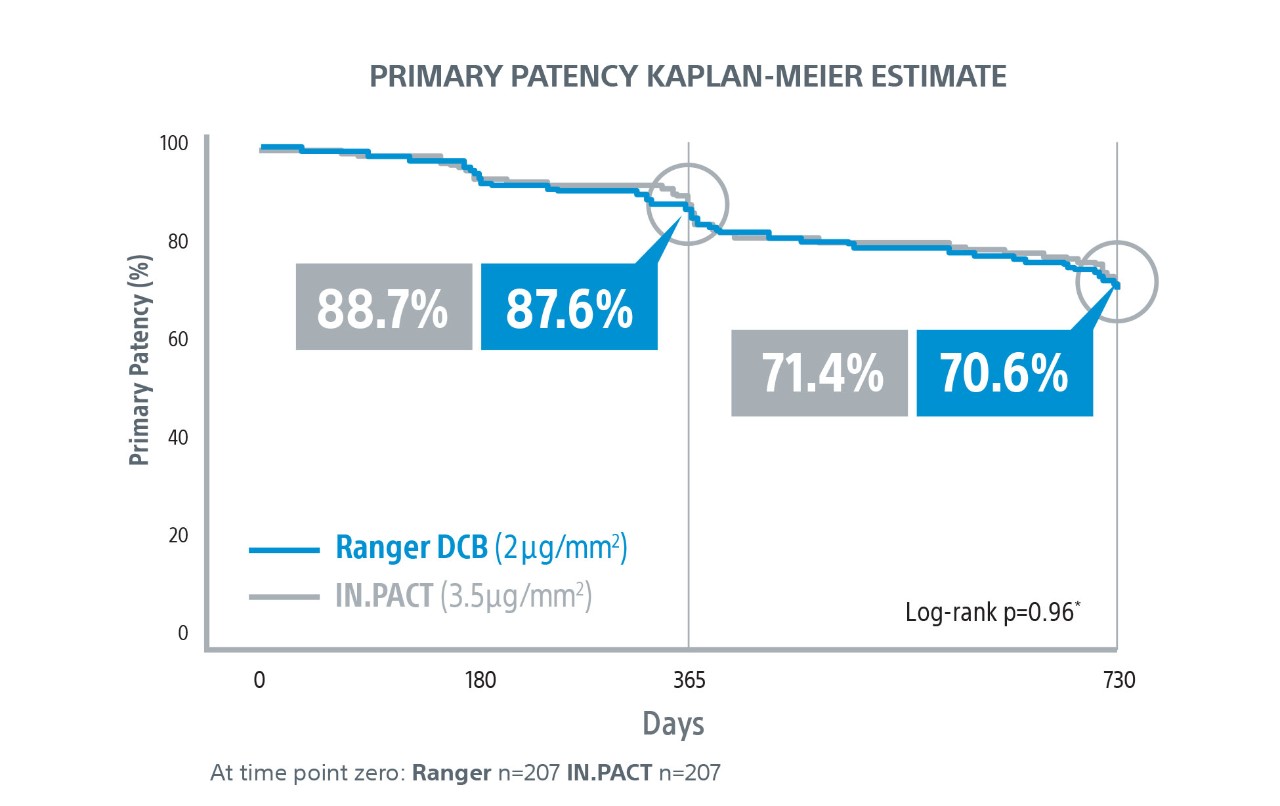
Ranger DCB demonstrated similar primary patency as IN.PACT at 2-Years¹ with half the total drug dose².
 BSC_DE_K-M_Chart_Ranger_v_IN.PACT_2_Year_V2
BSC_DE_K-M_Chart_Ranger_v_IN.PACT_2_Year_V2
*Log-rank p-value compares the entire K-M curves from time zero to full 2-Year follow-up window.
1. COMPARE Clinical Trial 2-Year Results presented by Sabine Steiner, MD. LINC 2021.
2. Based on total drug dose for (4mmx60mm) or (averages for full size matrix) per the Ranger™ Paclitaxel-Coated PTA Balloon Catheter and IN.PACT™ Admiral Instructions for Use.
COMPARE randomized controlled trial details
- 1-year primary endpoints
- Drug dose details
- Baseline characteristics
| Ranger DCB | IN.PACT | p-value | |
|---|---|---|---|
| Binary primary patency* | 83.0% (156/188) |
81.5% (141/173) |
P non-inferiority ‹0.01 |
| Freedom from major adverse events | 91.0% (182/200) |
92.6% (175/189) |
P non-inferiority ‹0.01 |
*Primary Endpoint Met.
| Ranger DCB (n=207) |
IN.PACT (n=207) |
p-value | |
|---|---|---|---|
| Excipient | TransPax™ citrate ester |
Urea | N/A |
| Paclitaxel dose density | 2.0 μg/mm2 | 3.5 μg/mm2 | N/A |
| Average total paclitaxel dose per patient in trial | 6,971 μg | 13,035 μg | ‹0.0001 |
| Ranger DCB (n=207) |
IN.PACT (n=207) |
p-value | |
|---|---|---|---|
| Age | 68.2 | 68.4 | 0.79 |
| Female | 38.2% | 36.2% | 0.68 |
| Current/Former Smoker | 77.3% | 75.3% | 0.63* |
| Total Occlusions | 41% | 43% | 0.62 |
| Total Occlusion Length | 131 mm | 113 mm | 0.23 |
| Target Lesion Length | 124 mm | 128 mm | 0.65 |
| Moderate to Severe Calcification** | 51% | 57% | *** |
| Diabetics | 31% | 37% | 0.18 |
* p-value based on entire distribution Never, Former or Current Smokers.
** PACSS Grade 3/4 may be considered similar to moderate/severe calcification.
*** p-value for entire distribution of PACSS Calcium Grades 0, 1, 2, 3, 4 calcium for RANGER DCB vs IN.PACT p-value was 0.20.
- 1-year key results
- 2-year key results
| Ranger DCB (n=207) |
IN.PACT (n=207) |
p-value | |
|---|---|---|---|
| Mortality: all cause | 2.5% | 1.6% | 0.73 |
| Mortality: device or procedure related | 0% | 0% | N/A |
| CD-TLR | 9.0% | 7.4% | 0.59 |
| Ranger DCB (n=207) |
IN.PACT (n=207) |
p-value | |
|---|---|---|---|
| Mortality: all cause | 3.6% | 2.2% | 0.6 |
| Mortality: device or procedure related | 0% | 0% | N/A |
| CD-TLR | 17.3% | 13.0% | 0.3 |
Primary safety endpoint: composite of freedom from device and procedure-related death through 30 days and freedom from major target limb amputation and CD-TLR through 1-Year post index-procedure.
Primary efficacy endpoint: primary patency at 1-Year defined as absence of clinically driven target lesion revascularization (CD-TLR) or binary restenosis determined as a peak systolic velocity ratio > 2.4 evaluated by duplex ultrasound core laboratory analysis.
CD-TLR: a reintervention performed for ≥ 50% diameter stenosis (confirmed by angiography) within ± 5 mm proximal and/or distal to the target lesion after documentation of recurrent clinical symptoms of PAD (increase of 1 Rutherford class or more) and/or drop of ABI (≥20% or >0.15 when compared to maximum early post-procedural level).
